Halogens

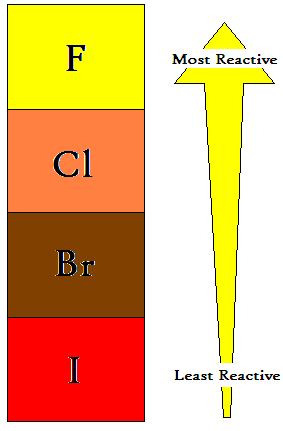 The reactive nonmetals that are in Group 17 of the periodic table. All of these elements are electronegative. The halogens show a number of trends when moving down the group - for instance, decreasing electronegativity and reactivity, increasing melting and boiling point. |
| Members of the Halogen group; Fluorine, Chlorine, Bromine, Iodine and Astatine. |
| The name "halogen" means "salt former"; halogens react with metals to form binary ionic compounds. At room temperature and pressure, fluorine andchlorine are gases, bromine is a liquid and iodine and astatine are solids; Group 7 is therefore the only periodic table group exhibiting all three states of matter. |
| Halogens are highly reactive, and as such can be harmful or lethal to biological organisms in sufficient quantities. This high reactivity is due to theiratoms being one electron short of a full outer shell of electrons. They can gain this electron by reacting with atoms of other elements. Fluorine is the most reactive element in existence, attacking such inert materials as glass, and forming compounds with the heavier noble gases. It is a corrosive and highly toxic gas. The reactivity of fluorine is such that, if used or stored in laboratory glassware, it can react with glass in the presence of small amounts of water to form SiF4. Thus fluorine must be handled with substances such as Teflon, extremely dry glass, or metals such as copper or steel which form a protective layer of fluoride on their surface. |
| Both chlorine and bromine are used as disinfectants for drinking water, swimming pools, fresh wounds, dishes, and surfaces. They kill bacteria and other potentially harmful microorganisms through a process known as sterilization. Their reactivity is also put to use in bleaching. Chlorine is the active ingredient of most fabric bleaches and is used in the production of most paper products. |

Post a Comment for "Halogens"