Energy Levels
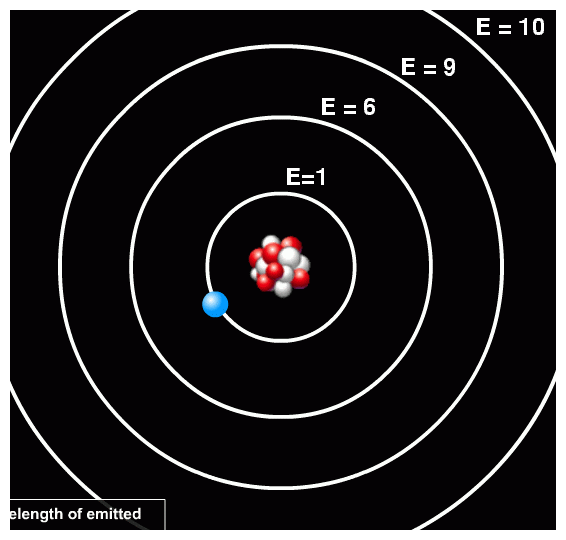 Electrons which surround the nucleus of the atom. Electrons are located in energy levels a term which has replaced the word shells, which was once used to describe the location of electrons. The word shell suggests a fixed position, which is far from reality. We will use energy level to describe the possible location of electrons. |
| There are seven energy levels. Each has a specific maximum number of electrons that can exist in it. The number of electrons, which an energy level can hold is equal to 2n2 where n = energy level. The letter n represents the principal quantum number that specifies the energy level of the atom in which an electron is located. The chart below identifies the various energy levels and maximum number of electrons possible. The energy level closest to the nucleus is represented by energy level 1. |

Post a Comment for "Energy Levels"