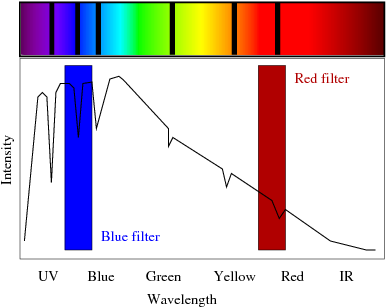
| A material's absorption spectrum shows the fraction of incident electromagnetic radiation absorbed by the material over a range of frequencies. An absorption spectrum is, in a sense, the opposite of an emission spectrum. Every chemical element has absorption lines at several particular wavelengths corresponding to the differences between the energy levels of its atomic orbitals. For example, an object that absorbs blue, green and yellow light will appear red when viewed under white light. Absorption spectra can therefore be used to identify elements present in a gas or liquid. |

Post a Comment for "Absorption Spectrum"