Predicting Metathesis Reactions (Chapter 4)
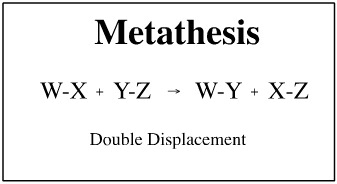
Metathesis or double replacement reactions take place when anions and cations of two salts change
partners. A metathesis reaction will occur if there is a net ionic equation.
This happens if (1) a precipitate forms from soluble reactants, (2) an
acid-base neutralization occurs, (3) a gas is formed, or (4) a weak
electrolytes. You should learn the solubility rules, and remember that all
salts are strong electrolytes. Remember that all strong acids and bases are
strong electrolytes, too. Strong acids react with strong bases in
neutralizations reactions to produce a salt and water. Acids react with
insoluble oxides and hydroxides to form water and the corresponding salt. Many
acid-base neutralization reactions can be viewed as a type of metathesis
reaction in which one product is water. Be sure to learn the reactions that
produce gases in metathesis reactions.

Source = Brady, James E. 2009. Chemistry Fifth Edition. Asia : John Willey & Sons

Source = Brady, James E. 2009. Chemistry Fifth Edition. Asia : John Willey & Sons

Post a Comment for "Predicting Metathesis Reactions (Chapter 4)"